Indian trials on multiple Covid-19 drugs make progress, have Atmanirbhar Bharat tilt
Mangalore Today News Network
New Delhi, Oct 21, 2020: At least two existing medicines that were repurposed for the treatment of coronavirus disease (Covid-19) have entered into phase III clinical trials that are being conducted by the Council for Scientific and Industrial Research (CSIR). This includes the antiviral medicine Umifenovir and a medicine that is used to treat blood infection caused by resistant gram-negative bacteria MW Sepsivac.
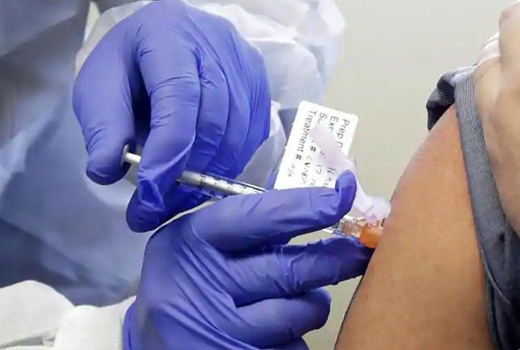
CSIR has also been working with phytopharmaceuticals – or herbal medicines – including an antiviral AQCH, which is about to enter phase III trial.
“We started a big clinical trial on MW Sepsivac as an immunomodulator and this trial has gone well. Phase II data has come out and now we are planning to launch the phase III trial. This immunomodulator will become a great support for Covid-19 patients. We are also working with phytopharmaceutical with AYUSH ministry. Today, our data for AQCH has come. This will be the first phytopharmaceutical to be evaluated with the modern trial approach,” said Dr S Chandrashekhar, director, Indian Institute of Chemical Technology (IICT) that is coordinating the trials.
It is also working on several combination therapies using antiviral drugs along with anti-gout medicine colchicine and a drug used to treat chest congestion called bromhexine. Approvals for phase I trial for these combination therapies have been received by the institute.
Dr Chandrashekhar was speaking on the occasion of the union health and science minister Harsh Vardhan launching the web portal for CSIR Ushered Repurposed Drugs (CuRED) that will provide updated information about drug trials and diagnostics being developed by CSIR laboratories.
“CSIR had started several trials for repurposed drugs to fight Covid-19 in collaboration with several companies. This portal will have information on these trials,” said Dr Shekhar Mande, DG, CSIR.
Apart from trials of repurposed drugs, IICT is also working on developing processes for at least 15 pharmaceutical compounds that are currently in trial across the world as possible therapy for Covid-19 but aren’t manufactured in India.
“Keeping the aim of Atmanirbhar Bharat in mind, we are also working on corticosteroids that are given to moderate and severe Covid-19 patients. However, we import 90% of these corticosteroids from China. Now multiple CSIR institutes are working not only to make these corticosteroids but another 15 API (active pharmaceutical ingredients) which are currently undergoing clinical trials for the treatment of Covid-19 across the world. We are sure in the next two to three months, we will be able to develop processes for 10 drugs and go to industrial partners for scale up and do clinical trials to show power of Indian generics,” said Dr Chandrashekhar.
As per the CuRED portal, two other testing kits for Covid-19 that have been developed by CSIR laboratories are currently under evaluation by the Indian Council of Medical Research (ICMR). One test named Feluda, which uses paper strip test developed by scientists from Institute of Genomics and Integrative Biology, has already been approved and is likely to hit the markets soon. The test barcodes the Cas9 protein – a component of the CRISPR gene editing system – to interact specifically with the genetic material of SARS-CoV-2 virus that causes Covid-19.
“So far, over 100 big or small technologies have been developed around Covid-19 by CSIR in the last nine months and most have been transferred to the industry. Finding a new molecule that specifically works against Covid-19 will take time, but in the meantime, scientists have been working with existing drugs to see whether some can be effective against Covid-19 such as Favipiravir that was used for flu in Japan or drugs that were used for Ebola. There are at least 25 drugs in various phases of trial and some have been successful. CSIR has also fulfilled my long-cherished dream that Ayurveda drugs be subjected to modern scientific scrutiny. All has happened in just nine months,” said union science minister Harsh Vardhan.
courtesy: Hindustan Times
- Kundapur: Boat goes off track as fishermen doze off
- Mangalore University hostel students protest against poor quality food
- Bonda factory gets clean chit in alleged food poisoning case
- Businessman succumbs to cardiac arrest while travelling in bus
- 123 couples tie the knot at mass marriage in Dharmasthala
- Mangaluru: CCB arrests two, seizes MDMA worth Rs 9 lakh
- Mangaluru :On May Day, TTEs stage protest at Central Railway station.
- Uppinangady: Woman missing with one year old daughter
- WhatsApp will soon block you from chatting temporarily if users violate its rules: All details
- Nasir murder case: Court sentences four convicts to life imprisonment
- Belthangady: Web cam stolen from polling booth at Thekkar
- Malpe: Life guards rescue 12 year boy from Chikkaballapura from drowning
- Man dies in freak mishap while tree felling
- PM Modi’s photo removed from CoWIN certificates due to model code of conduct
- Lookout notice issued against Prajwal Revanna over sex harassment videos
- Playback singer Uma Ramanan dies in Chennai
- Amid sex harassment charge, BJP’s Brij Bhushan, may be chosen in Polls
- Google lays off 200 ’core’ team employees, to shift jobs to India, Mexico: Report
- PM Modi’s 3rd term will eliminate terrorism from country: Amit Shah
- Heatwave red alert in six Karnataka districts as temperatures may cross 46 degrees Celsius
- Hindu marriage not valid unless performed with requisite ceremonies: SC
- GST revenues hit record ₹ 2.10 Lakh Crore in April. Here’s what led to it
- Deve Gowda planned escape of his grandson Prajwal, says Siddaramaiah
- Prajwal Revanna’s first reaction on sex tapes scandal: ’Truth will prevail soon’
- Accused in Salman Khan firing case dies after suicide attempt in police custody
- New residential complex for the judges inaugurated in Mangaluru
- Absconding accused nabbed after 8 years
- Truck with cylinders turns turtle in Beltangady
- Bhoota Kola artist dies of cardiac arrest
- Development of the country should be our goal: Ganesh Karnik
- Container truck gets stuck under Modankap railway bridge
- Truck crushes bike’s pillion rider near BC Road
- Head constable dies of heart attack
- Udupi: PDO dismissed over financial irregularities
- CREDAI to resume Skill Development Program for Construction Workers in Mangaluru
- John B Monteiro elected president of Rachana Catholic Chamber of Commerce & Industry
- Sudhanshu Rai elected district president of All College Student Association
- Chief Minister to visit Mangaluru, Udupi on August 1
- Nitte University awards PhD degree to Tina Sheetal D’Souza
- Sachitha Nandagopal honoured by CMTAI for Community Service
- CITY INFORMATION
- TRAVEL
- TOURIST INFORMATION
- HEALTH CARE
- MISCELLANEOUS




 Write Comment
Write Comment E-Mail To a Friend
E-Mail To a Friend Facebook
Facebook Twitter
Twitter  Print
Print 

